小社が立地する熊本にある熊本大学とパートナーシップ協定を結んでいるタイの大学研究者によるライフサイエンス分野の連載(4 回)をお届けします。
Exploiting Acquired Vulnerability to Develop Novel Treatments for Cholangiocarcinoma

Sirayot Areewong
Department of Pharmacology and Siriraj Center of Research Excellence for Cancer Precision Medicine & Systems Pharmacology,
Faculty of Medicine, Siriraj Hospital, Mahidol University

Sunisa Prasopporn
Department of Pharmacology and Siriraj Center of Research Excellence for Cancer Precision Medicine & Systems Pharmacology,
Faculty of Medicine, Siriraj Hospital, Mahidol University

Orawan Suppramote
Princess Srisavangavadhana College of Medicine, Chulabhorn Royal Academy

Siwanon Jirawatnotai
Department of Pharmacology and Siriraj Center of Research Excellence for Cancer Precision Medicine & Systems Pharmacology,
Faculty of Medicine, Siriraj Hospital, Mahidol University
Abstract
Cancer's adaptive capacity under therapeutic pressure constitutes a fundamental challenge in oncology, frequently culminating in treatment failure due to acquired resistance. Drug-induced adaptation plays a critical role in various malignancies, including colorectal, liver, glioma, and cholangiocarcinoma (CCA). A comprehensive characterization of cancer adaptation trajectories in response to specific therapeutic interventions enables the preemptive circumvention of resistance via rational drug combinations or the strategic exploitation of acquired vulnerabilities through synthetic lethality. Our laboratory is at the forefront of advancing this paradigm, particularly in the context of CCA — a highly lethal malignancy with significant prevalence in Thailand and East Asian countries. Through rigorous screening methodologies, we have systematically identified emergent vulnerabilities in drug-resistant CCA and validated these findings using tumoroid models and in vivo systems. Additionally, we have delineated key resistance trajectories under diverse treatment regimens, providing a mechanistic foundation for the development of novel therapeutic strategies. Our ongoing research program remains committed to elucidating the molecular determinants that govern CCA's response to pharmacological interventions. By integrating experimental insights with translational applications, we aim to contribute clinically actionable solutions that enhance therapeutic efficacy and improve patient outcomes in the management of this disease.
Overview of CCA as a Therapeutic Challenge
CCA is the second most common primary hepatic malignancy. Known for its extensive heterogeneity, silent clinical manifestations, and frequent late diagnosis, most patients are diagnosed with unresectable tumors. Current treatment options rely primarily on pharmacological approaches rather than surgery. Standard first-line therapies such as the gemcitabine/cisplatin (GEM/CIS) combination yield only modest improvements in overall survival (OS). Although efforts to enhance these results with additional agents (e.g., TS-1, nab-paclitaxel, or immune checkpoint inhibitors) have been made, the benefits remain limited. Second-line regimens, including FOLFOX (fluorouracil, oxaliplatin, and leucovorin), also only marginally extend survival, and targeted therapies addressing genetic mutations (e.g., FGFR2, IDH1/2) are applicable in only a small subset of patients1).
The Need for Novel Strategies
Due to CCA’s adaptability and rapid development of treatment resistance, there is an urgent need for alternative therapeutic paradigm. Instead of solely focusing on directly inhibiting oncogenic drivers or conventional survival pathways, we are exploring the concept of “acquired vulnerability.”2) This approach turns the cancer’s adaptive resistance into a therapeutic opportunity by targeting the weaknesses the cells acquire while evolving resistance3). These insights may lead to designing combination regimens capable of achieving markedly improved clinical outcomes.
Defining Acquired Vulnerability
When cancer cells are exposed to initial drug treatments, they undergo significant molecular reprogramming to counteract therapeutic pressure. In doing so, the cells “pay a price” for their resistance by developing new weaknesses — referred to as acquired vulnerabilities or collateral sensitivities4). Essentially, while resistant cells evade the effects of the primary drug, they become more susceptible to a secondary intervention that targets the reprogrammed survival mechanism (Figure 1).
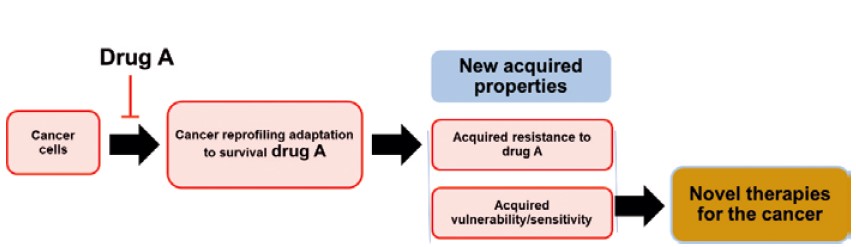
The concept of acquired vulnerability. Drug-naive cancer cells can develop resistance to drug A upon exposure. This resistance is accompanied by targetable molecular/pathway alterations, known as acquired vulnerabilities. These vulnerabilities may be exploited as novel therapies for the drug-resistance cancer in two ways: (1) by targeting acquired dependencies on survival mechanisms induced by drug A or (2) by targeting acquired decompensations where cancer cells cannot fully mitigate drug A-induced damage
Historical Context and Early Observations
Early studies dating back to the 1950s noted that resistance to one drug could lead to increased sensitivity to another. Initial work in leukemia models and subsequent microbial studies, like those by Szybalski and colleagues, established the principle of “collateral sensitivity.”4) We expand upon these early observations by applying the concept of acquired vulnerability to cholangiocarcinoma, demonstrating how treatment-induced adaptations create new targetable weaknesses.
Mechanistic Underpinnings4)
Unlike classic genetic resistance mechanisms, which can be detected by DNA sequencing, acquired vulnerability typically arises from non-genetic adaptations — including altered gene expression, posttranslational modifications, and reorganization of cellular signaling networks. These changes force the cancer cells to become “addicted” to the alternative survival pathways they establish under treatment pressure, thereby opening a window for targeted secondary therapies.
Diverse Mechanistic Categories which cancer acquired vulnerabilities manifest:
1. Apoptotic Pathway Dysregulation:
Resistant cells may downregulate pro-apoptosis or exhibit upregulation of inhibitors of apoptosis proteins (IAPs). Such reprogramming can be exploited with agents like TRAIL receptor agonists or SMAC mimetics that induce apoptosis.
2. Cell Cycle Dysregulation:
Alterations such as the loss of CDKN2A or the overexpression of cyclins (e.g., cyclin D1) force cells to depend on compensatory cell cycle pathways. Combining CDK inhibitors with agents targeting these compensatory mechanisms has been shown to induce effective cell death.
3. Growth Signaling Rewiring:
When cells inhibit their primary survival pathways (e.g., receptor tyrosine kinases or MAPK pathways), they often become reliant on alternative pathways such as the PI3K/AKT/mTOR axis. This dependency can be targeted using specific inhibitors.
4. Oxidative and Metabolic Stress:
Many resistant cancer cells modulate their levels of reactive oxygen species (ROS) by altering antioxidant responses or metabolic pathways. Agents that either further disrupt this balance or force a metabolic shift (e.g., from glycolysis to mitochondrial oxidative phosphorylation) can trigger lethal oxidative damage.
5. Protein Synthesis and Ribosomal Stress:
In response to certain inhibitors (e.g., CDK4/6 inhibitors), resistant cholangiocarcinoma cells may increase ribosomal biogenesis factors, making them particularly vulnerable to drugs that impair nucleolar function (e.g., oxaliplatin).
6. Hypoxic and DNA Repair Stress:
Prolonged treatment can induce dependencies on specific DNA repair mechanisms (such as the ATR/CHK1 pathway) or lead to intracellular hypoxia. Inhibiting these pathways can result in catastrophic DNA damage and cell death.
Identification Strategies and Combination Therapeutic Approaches4)
The identification of these vulnerabilities relies on a combination of comparative drug screening and high-throughput molecular analyses:
- Comparative Drug Screening:
Researchers generate resistant cell clones by exposing CCA cell lines to primary drugs (e.g., GEM/CIS). These resistant clones are then compared alongside drug-naïve cells using a broad library of compounds. Agents that selectively impair the resistant cells are flagged as potential secondary treatments. - Omics and Molecular Analyses:
Techniques such as proteomics, transcriptomics, and chromatin accessibility assays help elucidate the reprogrammed signaling networks. This process not only identifies critical pathways but also aids in discovering surrogate biomarkers that could be used clinically for patient stratification.
Validation Using Advanced Models
For clinical translation, findings from in vitro screens are validated using patient-derived organoids (PDOs) and patient-derived xenografts (PDXs), which serve as “avatar” models. These models capture the unique heterogeneity of individual tumors, enabling personalized validation of drug combinations and adjustments for each patient’s specific tumor biology.
Combination Strategies and Regimen Scheduling
A key insight is that exploiting acquired vulnerabilities is most effective when secondary treatments are combined with the primary therapy rather than being used alone. Different scheduling approaches are considered:
- Sequential Dosing: Secondary agents are introduced only after resistance develops.
- Preconcomitant Dosing: Secondary agents are administrated at the outset alongside the primary drug to preempt resistance.
- Postconcomitant Dosing: Combination therapy is initiated only after the resistance phenotype is observed.
Continuous exposure to the primary therapy may be necessary to maintain the vulnerability, as intermittent dosing or prolonged drug holidays might allow cancer cells to revert to their original state.
Discoveries of the acquired vulnerability-targeting therapy in CCA
We have demonstrated distinguish targeting strategies for the CCA that developed drug resistance to the first-line treatment gemcitabine + cisplatin (GEM/CIS) and the CDK4/6 inhibitor.
SMAC Mimetic and GEM/CIS in CCA5)
We examined CCA cells that had acquired resistance to the standard GEM/CIS regimen. We observed that prolonged chemotherapy exposure led to upregulation of the anti-apoptotic protein cIAP2 — a key regulator in the inhibition of apoptosis. This reprogramming, while conferring resistance to GEM/CIS, simultaneously created a susceptibility to agents targeting cIAP2. Treatment with the SMAC mimetic LCL161 effectively downregulated cIAP2 levels. When combined with GEM/CIS, LCL161 not only restored sensitivity in resistant CCA cell lines but also produced a strong synergistic effect in both in vitro experiments and in vivo xenograft models. This combination inhibited the emergence of further multidrug resistance,suggesting that exploiting the cancer’s compensatory upregulation of anti-apoptotic proteins may enhance the efficacy of standard chemotherapy.
CDK4/6 Inhibition – Induced Vulnerability Exploited by Oxaliplatin/Palbociclib combination6)
The second study focused on an alternative approach:inducing an acquired vulnerability by inhibiting CDK4/6. Treatment of CCA cells with a CDK4/6 inhibitor (palbociclib) resulted in adaptive reprogramming characterized by significant overexpression of the ribosomal protein RPL29. This elevation of RPL29 is associated with the resistance to CDK4/6 inhibition. However, this same reprogramming creates a dependency on the ribosomal biogenesis pathway — a vulnerability that can be exploited. When oxaliplatin, an agent known to disrupt nucleolar function and protein synthesis, was combined with palbociclib, the treatment induced RPL29 suppression. This triggered a cascade involving RPL5/11-mediated inhibition of MDM2, stabilization of p53, and ultimately, cancer cell apoptosis. The combination of oxaliplatin with palbociclib cleared resistant cells more effectively than when the drugs were used separately, highlighting the potential of leveraging an induced ribosomal stress vulnerability to overcome drug resistance.
Both of our studies highlighted a conceptual shift in cancer treatment strategies. They illustrate that the very adaptations cancer cells use to evade therapies can be exploited as weaknesses.vThese complementary approaches support the concept of “acquired vulnerability” as a means of designing combination regimens. Rather than considering drug resistance as an insurmountable barrier, these findings suggest that it represents an opportunity to intervene with secondary agents that specifically target the reprogrammed survival mechanisms in resistant cells. Both investigations relied on comprehensive in vitro assays, xenograft models, and — where available — patient-derived tumoroids/organoids to validate their observations. Their preclinical success paves the way for future clinical trials which might incorporate these drug combinations into treatment protocols for patients with CCA.
Challenges and Future Directions
Although the concept of acquired vulnerability is both innovative and promising, several challenges must be addressed before such strategies can be widely implemented in the clinic: Biomarker Development:
Acquired vulnerabilities often arise from non-genetic changes. Therefore, conventional genomic tests may be insufficient, and the discovery of reliable biomarkers is essential for proper patient selection and treatment monitoring. Tumor Heterogeneity:
Inherent variability within and between tumors makes the application of a single strategy difficult. Personalized approaches using PDOs or PDXs are necessary to tailor treatments, although this can complicate clinical trial designs. Optimization of Drug Combinations:
While preclinical studies have identified promising combinations that demonstrate synergy, translating these findings into the clinical setting requires careful balancing to reduce toxicity while maximizing efficacy. Dynamic Nature of Vulnerability: Acquired vulnerability is not a permanent trait; it can fluctuate or disappear if the selective pressure of the primary therapy is removed. Maintaining this vulnerability through continuous or appropriately scheduled dosing is critical.
We envision an integrated strategy where innovative technologies — such as single-cell sequencing and cellular barcoding — are used to further elucidate and track acquired vulnerabilities over time. Expanding research into additional aspects of tumor biology, including epigenetic modifications and microenvironment interactions, may unlock new therapeutic targets. Well-designed clinical trials that incorporate adaptive treatment approaches based on these evolving vulnerabilities hold the potential for significantly improved patient outcomes.
Conclusion
We redefine drug resistance in CCA by demonstrating that the very process of acquiring resistance leads to the emergence of exploitable weaknesses. By classifying acquired vulnerabilities — from apoptotic and cell cycle dysregulation to metabolic reprogramming and ribosomal stress — and outlining robust strategies for their detection and exploitation, the paper sets a comprehensive framework for developing novel, adaptive combination therapies. Although challenges remain, the potential to transform management not only for CCA but for other treatment-resistant cancers is compelling, heralding a promising new direction in personalized oncology.
This paradigm shift — moving from a singular focus on genetic mutations to dynamically targeting adaptive cellular changes — could revolutionize how we treat various cancers. With continuous monitoring and adaptive drug scheduling, the approach of exploiting acquired vulnerability may lead to more durable responses and improved survival outcomes for patients facing aggressive and heterogeneous malignancies.
[References]
- J. M. Banales, J. J. G. Marin, A. Lamarca, P. M. Rodrigues, S. A. Khan, L. R. Roberts et al., “Cholangiocarcinoma 2020: the next horizon in mechanisms and management”, Nat. Rev. Gastroenterol Hepatol., 2020, 17(9), 557–588.
- L. Wang and R. Bernards, “Taking advantage of drug resistance, a new approach in the war on cancer”, Front. Med., 2018, 12(4), 490–495.
- M. H. Dias, C. Papagianni and R. Bernards, “The case for therapeutic overactivation of oncogenic signaling as a potential cancer treatment strategy”, Cancer Cell, 2024, 42(6), 919–922.
- S. Areewong, O. Suppramote, S. Prasopporn and S. Jirawatnotai, “Exploiting acquired vulnerability to develop novel treatments for cholangiocarcinoma”, Cancer Cell Int., 2024, 24(1), 362.
- S. Prasopporn, O. Suppramote, B. Ponvilawan, C. Jamyuang, J. Chanthercrob, A. Chaiboonchoe et al., “Combining the SMAC mimetic LCL161 with Gemcitabine plus Cisplatin therapy inhibits and prevents the emergence of multidrug resistance in cholangiocarcinoma”, Front. Oncol., 2022, 12, 1021632.
- O. Suppramote, S. Prasopporn, S. Aroonpruksakul, B. Ponvilawan, J. Makjaroen, M. Suntiparpluacha et al., “The Acquired Vulnerability Caused by CDK4/6 Inhibition Promotes Drug Synergism Between Oxaliplatin and Palbociclib in Cholangiocarcinoma”, Front. Oncol., 2022, 12, 877194.
[Contact]
Sirayot Areewong
Department of Pharmacology and Siriraj Center of Research Excellence for Cancer Precision Medicine & Systems Pharmacology, Faculty of Medicine, Siriraj Hospital, Mahidol University, 10700, Bangkok, Thailand
Current research area: Acquired drug resistance in cancer
Sunisa Prasopporn
Department of Pharmacology and Siriraj Center of Research Excellence for Cancer Precision Medicine & Systems Pharmacology, Faculty of Medicine, Siriraj Hospital, Mahidol University, 10700, Bangkok, Thailand
Current research area: Cancer acquired drug resistance, epigenetic controls in cancer cell, in vivo cancer models, cholangiocarcinoma
Orawan Suppramote
Princess Srisavangavadhana College of Medicine, Chulabhorn Royal Academy, 906 Kampangpetch 6 Rd., Talat Bang Khen, Lak Si, 10210, Bangkok, Thailand.
Current research area: Molecular oncology, systems pharmacology, proteomics
Siwanon Jirawatnotai
Department of Pharmacology and Siriraj Center of Research Excellence for Cancer Precision Medicine & Systems Pharmacology, Faculty of Medicine, Siriraj Hospital, Mahidol University, 10700, Bangkok, Thailand
Current research area: Cancer acquired drug resistance, cell cycle regulations in cancer, cholangiocarcinoma, cancer genetics, epigenetic controls in cancer cell, cancer precision medicine
