Detecting ferroptosis: Tools, Markers, and Applications

Jiewen Zheng
Department of Chemistry,
Columbia University

Brent R. Stockwell
Department of Chemistry,
Columbia University
Department of Biological Sciences,
Columbia University
Irving Institute for Cancer Dynamics,
Columbia University
Herbert Irving Comprehensive Cancer
Center, Columbia University
Data Science Institute, Columbia University
Department of Pathology and Cell Biology
and Columbia University Digestive and
Liver Disease Research Center, Vagelos
College of Physicians and Surgeons,
Columbia University Irving Medical Center
Abstract Ferroptosis, a non-apoptotic cell death driven by iron-dependent phospholipid peroxidation, has been implicated in various biological contexts. It is important to develop reliable tools to detect and monitor ferroptosis in both in vitro and in vivo systems. In this review, we highlight current biochemical and immunodetection strategies used to identify ferroptosis and the organelle-specific regulators. We also outline the emerging technologies and future directions to advance ferroptosis research and their therapeutic and diagnostic potentials.
1.Introduction
Ferroptosis is a distinct form of regulated cell death driven by iron-dependent accumulation of peroxidized phospholipids, particularly those containing polyunsaturated fatty acyl (PUFA) tails1). Unlike apoptosis, necroptosis, or pyroptosis, ferroptosis is triggered by the disruption of antioxidant defense systems, most notably the glutathione peroxidase 4 (GPX4) axis, leading to oxidative damage of cellular membranes and ultimately cell death2),3).
Over the past decade, ferroptosis has emerged as a critical mechanism in both physiological and pathological contexts, including cancers, immune disorders, neurodegeneration, and aging4). The regulatory network underlying ferroptosis involves the crosstalk among iron metabolism, lipid peroxidation, and antioxidant defenses. Importantly, the discovery of small-molecule or natural products regulating ferroptosis has unveiled the therapeutic potential of targeting ferroptosis in disease models5). For example, the ferroptosis inducer imidazole ketone erastin (IKE), which selectively inhibits the cystine-glutamate antiporter system Xc-, demonstrates anti-tumor effects in lymphoma models6). In contrast, inhibition of ferroptosis using radical-trapping antioxidant agents, such as liproxstatin-1, significantly attenuates disease progression in mouse models of disease, underscoring its potential in autoimmune and other disease indications7).
Given the growing therapeutic relevance of ferroptosis, there is a need for the development of robust, sensitive, and specific tools to detect and monitor ferroptosis in both in vitro and in vivo. Current methods include biochemical assays for lipid peroxidation and labile iron accumulation, as well as immunodetection of ferroptosis-associated proteins such as GPX4, Acyl-CoA Synthetase Long Chain Family Member 4 (ACSL4), and transferrin receptor 1 (TfR1)8). Moreover, the integration of nanotechnology and ferroptosis detection strategies has opened avenues for the mechanistic investigation of ferroptosis at the nanoscale and for clinical applications9).
Here, we provide a focused overview of current methodologies for detecting ferroptosis. We highlight key tools, including chemical probes, immunodetection strategies, and organelle-specific approaches, and emerging biomarkers and their applications in disease models. We also discuss how ferroptosis can be detected in specific pathophysiological contexts and conclude by addressing the limitations and opportunities for standardizing ferroptosis detection in basic and translational research.
2.Methods and Markers for Detecting Ferroptosis
2.1. Biochemical Detection of Ferroptosis
Lipid peroxidation is a defining biochemical feature of ferroptosis and plays a central role in modulating ferroptosis. The accumulation of lipid-based reactive oxygen species (ROS), particularly lipid hydroperoxides, is a key indicator of ferroptotic cell death10). Several detection platforms have been developed to detect and quantify the extent of lipid peroxidation in cellular and tissue systems undergoing ferroptosis.
One useful probe is C11-BODIPY581/591, a lipophilic fluorescent dye that localizes into membranes and shifts its fluorescence emission from red (598nm) to green (520nm) upon oxidation11). This ratiometric change can be quantified by flow cytometry or live-cell imaging, offering a real-time readout of lipid peroxidation. However, C11-BODIPY581/591 has limited selectivity to lipid hydroperoxides relevant to ferroptosis.
To address this limitation, Liperfluo, a recently developed lipid hydroperoxide-selective probe, offers potentially improved specificity for ferroptosis2). Unlike C11-BODIPY, Liperfluo reacts directly with lipid hydroperoxides, while showing minimal cross-reactivity with general ROS or hydrogen peroxide. Liperfluo is
complementary and potentially advantageous due to its improved selectivity, lower background, and minimal interference from other oxidative species. It can be used in both fixed and live cells and is compatible with high-content and confocal imaging12).
For a more comprehensive assessment of oxidized lipid species, oxidative lipidomics is a powerful technique. Mass spectrometry-based approaches, such as liquid chromatography with tandem mass spectrometry (LC-MS/MS), enable the precise, high-resolution identification and quantification of oxidized lipid species, particularly oxidized phosphatidylethanolamines (PEs), the major pro-ferroptotic lipid class13). These approaches enable the detection of subtle lipidomic changes associated with ferroptosis in both in vitro and in vivo models and can be further extended to profile oxidative protein modifications in parallel2).
Iron overload contributes to ferroptosis through the Fenton reaction, which generates ROS that propagate lipid peroxidation14). Labile iron pools can be detected using fluorescent probes such as FerroOrange, a selective cytosolic Fe2+ indicator. This probe allows for flow cytometry, live-cell imaging and quantification of intracellular iron levels. We treated SU-DHL-5 cells with a GPX4 inhibitor, RSL3, and measured labile Fe2+. We observed that RSL3 treatment significantly increased the accumulation of labile Fe2+ level, evidenced by a marked increase in FerroOrange fluorescence in flow cytometry measurement (Figure 1). An alternative method using Calcein-AM fluorescence quenching can also measure the labile iron pool15). This method relies on the principle that Fe2+ quenches calcein fluorescence, allowing quantification of the labile iron pool. These complementary techniques provide critical insight into the iron driving lipid peroxidation and ferroptosis.
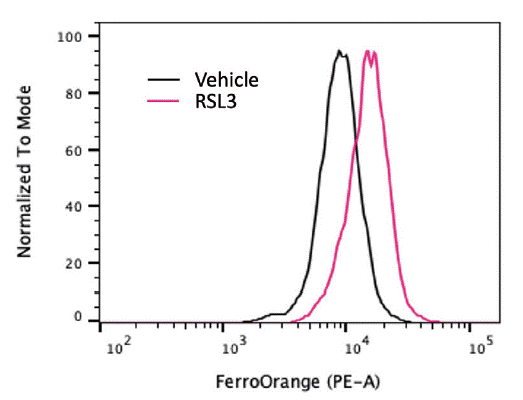
Labile Fe2+ measured by FerroOrange in SU-DHL-5 cells treated with vehicle or 10 μM RSL3 for 6 hr.
2.2. Organelles Specific Observations in Ferroptosis
Ferroptosis is a multifaceted process involving lipid metabolism, redox balance, and iron homeostasis across multiple subcellular compartments. Rather than occurring in isolation, ferroptosis involves dynamic interactions among organelles that collectively shape the cell’s vulnerability to lipid peroxidation.
Mitochondria contribute to ferroptosis, including metabolic reactions, iron handling, stress response, and the biosynthesis of coenzyme Q10 (CoQ10), a critical antioxidant and electron carrier16). Morphological hallmarks such as cristae shrinkage and outer membrane rupture are commonly detectable by transmission electron microscopy (TEM)1).
The endoplasmic reticulum (ER) serves as a central hub for lipid biosynthesis, including lipid desaturation, phospholipid synthesis, and lipid remodelling. Key lipid metabolic enzymes ACSL4 and lysophosphatidylcholine acyltransferase 3 (LPCAT3), localized to the ER, enable the incorporation of PUFAs into phospholipids, which are key substrates for lipid peroxidation16). In addition, the ER membrane is a key site of lipid peroxidation, and initially accumulates membrane peroxidation, which can then propagate to the plasma membrane17).
Lipid droplets (LDs), which are derived from the ER, regulate ferroptosis through lipid storage and trafficking. LDs have the unique ability to sequester excess PUFAs by esterifying them into triacylglycerols (TAGs), thereby protecting cells against lipid toxicity and ferroptosis18). Moreover, the sequestration of autophagy-derived FAs in LDs also enables a precise regulation of FA-delivery to mitochondria through the lipolytic activity of adipose triglyceride lipase (ATGL)19). Dysregulation of this ATGL-dependent lipolytic pathway sensitizes cells to ferroptosis by enhancing the release of peroxidation-prone lipids.
Together, these organelle-specific events highlight the spatial complexity of ferroptosis regulation and offer insights for targeted detection and intervention strategies.
2.3. Immunodetection for Ferroptosis Markers
In addition to biochemical assays, immunodetection techniques offer essential tools for ferroptosis detection by targeting key protein markers involved in its regulation and execution. These techniques, including immunoblotting, immunofluorescence (IF), and immunohistochemistry (IHC), allow for the spatial and quantitative analysis of ferroptosis-associated protein markers in cells and tissues.
The enzyme GPX4, which reduces phospholipid hydroperoxides to non-lethal lipid alcohols using glutathione (GSH) as a cofactor, acts as an important protector against ferroptosis20). Therefore, loss or functional impairment of GPX4 can drive ferroptosis. Antibodies targeting GPX4 have been used for western blotting, IF, and IHC staining to quantify the level of the protein21). Moreover, since the expression and activity of GPX4 in ferroptosis rely on the system Xc--mediated supply of the cysteine/cystine pool and subsequent GSH biosynthesis, regulation of SLC7A11, a component of the system Xc- antiporter, has been used as a ferroptosis marker in some models22).
ACSL4 is a critical protein implicated in ferroptosis sensitivity. ACSL4 facilitates the incorporation of PUFAs into phospholipids, thereby enriching cells with substrates for lipid peroxidation. Upregulation of ACSL4 promotes ferroptosis, while reduction of ACSL4 abundance confers resistance23). Antibodies against ACSL4 have been used in immunoblotting and immunostaining to assess ferroptosis susceptibility.
In addition, TfR1, an iron import protein, is frequently upregulated during ferroptosis due to increased iron demand and altered iron homeostasis. A recent study validated that an antibody targeting TfR1 is effective as a selective ferroptosis-staining reagent24).
Other immunodetection markers include hyperoxidized peroxiredoxin 3 (PRDX3), a mitochondrial peroxidase whose oxidation may reflect mitochondrial oxidative injury during ferroptosis25), and malondialdehyde (MDA), a reactive aldehyde byproduct of lipid peroxidation, can be detected in ferroptotic cells using IF, IHC, or ELISA26).
These immunodetection strategies provide complementary evidence for ferroptosis, particularly in in vivo studies and clinical samples, where lipid peroxidation probes may be limited by tissue penetration or specificity.
We summarize key ferroptosis detection methods, including biochemical detection, immunodetection, and other imaging methods, in Table 1. This overview serves as a practical guide for selecting appropriate tools in various experimental and translational contexts.
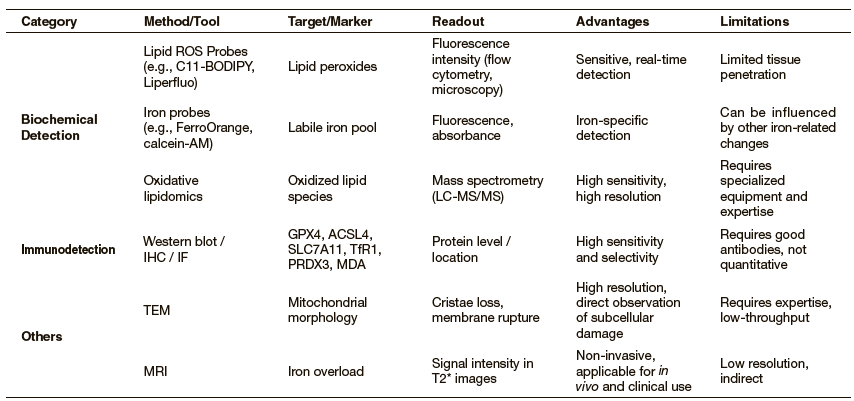
Note: ROS: reactive oxygen species; LC-MS/MS: liquid chromatography with tandem mass spectrometry; IHC: immunohistochemistry; IF: immunofluorescence;
TEM: transmission electron microscopy; MRI: magnetic resonance imaging.
3.Disease Contexts and Translational Applications
Ferroptosis plays diverse roles in different pathological contexts, particularly in diseases characterized by dysregulated lipid metabolism, redox imbalance, and iron overload27). In cancer treatment approaches, particularly for therapy-resistant cancer types and diffuse large B-cell lymphoma, ferroptosis represents a potential therapeutic vulnerability6). In contrast, excessive ferroptosis contributes to cell death in neurodegenerative diseases such as Alzheimer's, Parkinson’s, and Huntington's disease with iron overload28). Ferroptosis is also involved in acute injuries like ischemia-reperfusion injury (IRI) in the heart, liver, and kidney, where Wnt signaling-mediated oxidative stress bursts upon reperfusion29).
Ferroptosis offers novel therapeutic and diagnostic opportunities. Ferroptosis inducers, such as IKE and cystine/methionine deprivation, show antitumor effects in animal models, while inhibitors protect against neurodegeneration and IRI29),30). Diagnostic applications are also improving, whereby immunostaining for ferroptosis markers, including TfR1, MDA, and hyperoxidized PRDX3, aids in tissue-based diagnostics; radiological tools, such as MRI-based iron imaging, can detect iron accumulation in organs31). Additionally, ferroptosis-related lipidomic signatures and protein markers hold promise as prognostic and predictive biomarkers23). Together, these therapeutic and diagnostic strategies highlight the clinical relevance of ferroptosis in diverse pathological settings.
4.Challenges and Future Directions
We have witnessed the development of techniques in understanding ferroptosis and their potential in clinical applications. However, there are limitations in the specificity and sensitivity of current methods. For example, lipid peroxidation byproducts and antioxidant proteins overlap with other oxidative stress pathways32). Also, since redox conditions vary between cell-based cultures and in vivo conditions, there is a lack of reliable ferroptosis-detecting tools for in vivo and tissue samples14). Moreover, heterogeneity of ferroptosis regulation across cell types and disease contexts complicates therapeutic targeting, suggesting the necessity of personalized approaches.
Integrating multi-omic profiling, advanced imaging modalities, and organelle-specific markers will enhance the understanding of ferroptosis. Developing selective probes and therapeutics that target key ferroptosis regulators with high efficacy and low toxicity is critical. Finally, interdisciplinary efforts combining systems biology, nanotechnology, and clinical expertise will be essential to utilize ferroptosis as a diagnostic and therapeutic avenue in cancer and other acute settings.
[Contact]
Jiewen Zheng (PhD student)
Current research area: Ferroptosis, Lipid Metabolism, Diet, Cancer Biology
Brent R. Stockwell (Corresponding author)
Current research area: Ferroptosis, Lipid Metabolism, Diet, Cancer Biology, Chemical Biology, Mass Spectrometry, Neurodegeneration