The Past Half-Century of Regulated Cell Death Research
Dojindo Laboratories, Chaojun Zhang (張超俊)
The study of regulated cell death is central to understanding many diseases, including cancer and neurodegenerative diseases. In the past half-century, the knowledge gained on regulated cell death has resulted in the discovery of numerous new cell-death mechanisms. Dojindo Laboratories have focused on cell biology in the past several decades, and the company has developed many products related to regulated cell death to cater to the needs of cutting-edge research. The upper half of Figure 1 shows the history of regulated cell death, and the lower half displays the new research tools developed or commercialized by Dojindo Laboratories.
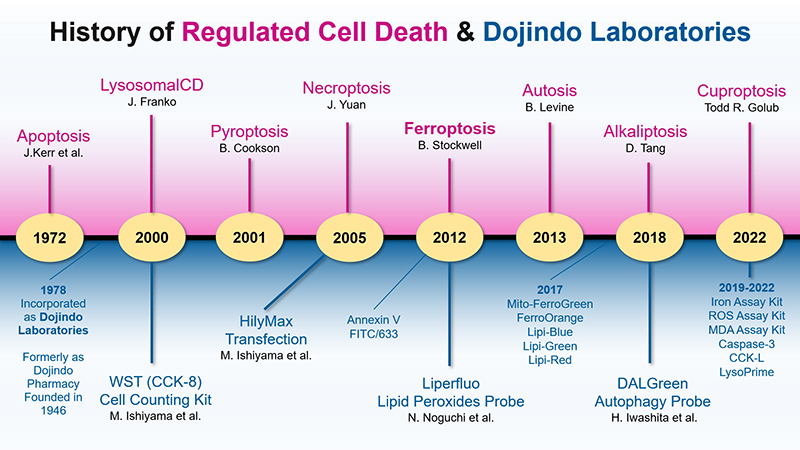
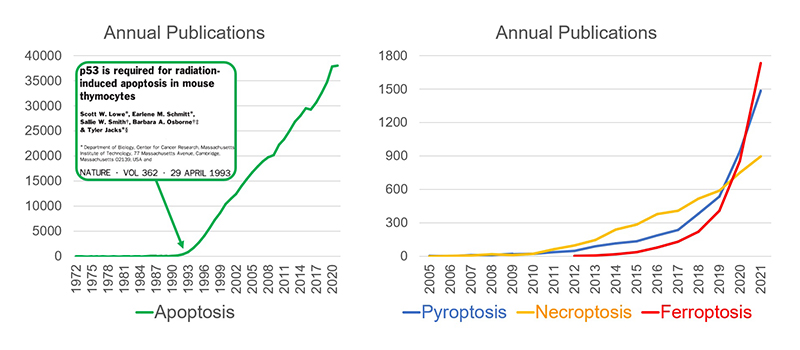
In 1972, John Kerr from the University of Queensland and his colleagues published a seminal article on cell death in the British Journal of Cancer. In the article, natural cell death was called apoptosis for the first time1). After that, many regulated cell death types, including pyroptosis in 2001, necroptosis in 2005, ferroptosis in 2012, and autosis in 2013, have been recognized by the scientific community. The most widely studied type is undoubtedly apoptosis2). However, in the first 20 years after its discovery, apoptosis did not receive much attention, as demonstrated by the number of annual publications (Figure 2, left). In 1993, with the discovery of an association between apoptosis and p53, the well-known tumor suppressor gene3), there was explosive growth in the number of annual apoptosis-related publications that continues to the present day (Data Source: PubMed).
Although ferroptosis is one of the more recent discoveries, the growth in ferroptosis papers has surpassed that of necroptosis and pyroptosis, which were respectively discovered in 2001 and 2005 (Figure 2, right). It appears that only ferroptosis has experienced a similar publication growth rate to apoptosis.
Ferroptosis, a unique iron-dependent form of nonapoptotic cell death associated with increased lipid peroxides, was named by Professor Stockwell in 20124).
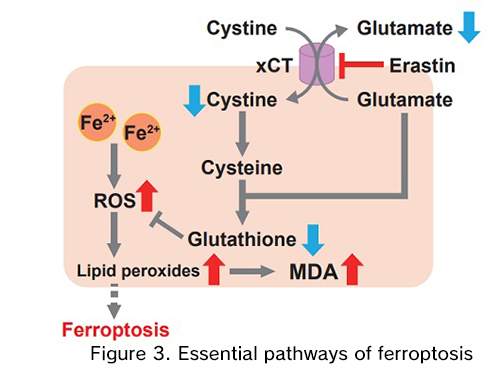 Small molecules such as erastin and (1S, 3R)-RSL3 are known inhibitors of tumor cell growth that induce ferroptosis. These compounds do not trigger apoptosis and therefore do not cause chromatin margination or polymerase cleavage. Instead, ferroptosis causes changes in the mitochondrial phenotype. Iron is also necessary for small-molecule ferroptosis induction; therefore, these inhibitors can be inhibited by iron chelators. Erastin acts through the inhibition of the cystine/glutamate transporter, causing decreased intracellular glutathione (GSH) levels5). Given that GSH is necessary for GPX4 function, depletion of this cofactor can lead to ferroptotic cell death. Ferroptosis can also be induced through the inhibition of GPX4, which is the molecular mechanism of action of RSL3.
Small molecules such as erastin and (1S, 3R)-RSL3 are known inhibitors of tumor cell growth that induce ferroptosis. These compounds do not trigger apoptosis and therefore do not cause chromatin margination or polymerase cleavage. Instead, ferroptosis causes changes in the mitochondrial phenotype. Iron is also necessary for small-molecule ferroptosis induction; therefore, these inhibitors can be inhibited by iron chelators. Erastin acts through the inhibition of the cystine/glutamate transporter, causing decreased intracellular glutathione (GSH) levels5). Given that GSH is necessary for GPX4 function, depletion of this cofactor can lead to ferroptotic cell death. Ferroptosis can also be induced through the inhibition of GPX4, which is the molecular mechanism of action of RSL3.
 In October 2022, the Iron, ROS & Ferroptosis in Life, Death & Disease Conference was held in Awaji Island, Japan. Hundreds of ferroptosis researchers from institutions worldwide attended the conference, including Professor Stockwell, who gave a keynote speech titled “10 years of ferroptosis—Emerging mechanisms and therapeutic applications”. In his speech, he summarized key pathological contexts involving ferroptosis and emerging therapeutic applications that modulate ferroptosis. Dojindo Laboratories also supported and attended this conference.
In October 2022, the Iron, ROS & Ferroptosis in Life, Death & Disease Conference was held in Awaji Island, Japan. Hundreds of ferroptosis researchers from institutions worldwide attended the conference, including Professor Stockwell, who gave a keynote speech titled “10 years of ferroptosis—Emerging mechanisms and therapeutic applications”. In his speech, he summarized key pathological contexts involving ferroptosis and emerging therapeutic applications that modulate ferroptosis. Dojindo Laboratories also supported and attended this conference.
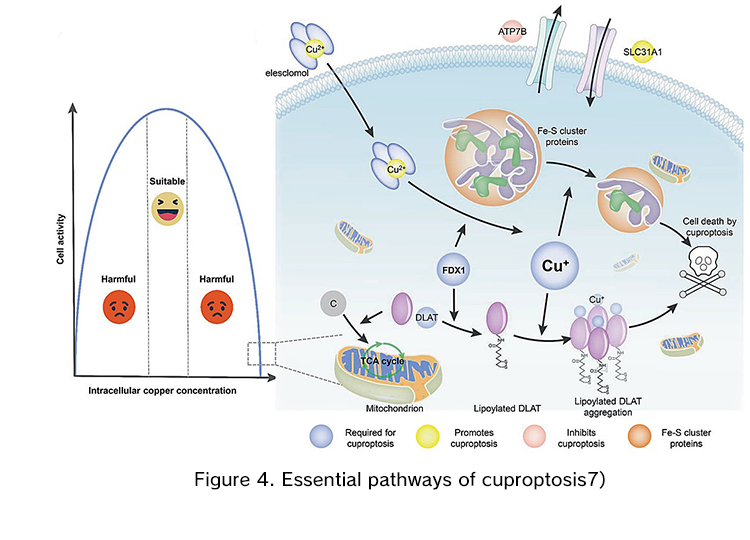 Recently Todd Golub's team reported a new copper-ion-dependent form of cell death called cuproptosis6). They showed that copper-dependent regulated cell death in human cells is dependent on mitochondrial respiration and occurs by means of direct binding between copper and lipoylated components of the TCA cycle. This results in lipoylated protein aggregation and subsequent iron-sulfur cluster protein loss, which leads to proteotoxic stress and ultimately cell death. To better demonstrate the cuproptosis pathway, an illustration from a recently published paper is provided in Figure 4 7).
Recently Todd Golub's team reported a new copper-ion-dependent form of cell death called cuproptosis6). They showed that copper-dependent regulated cell death in human cells is dependent on mitochondrial respiration and occurs by means of direct binding between copper and lipoylated components of the TCA cycle. This results in lipoylated protein aggregation and subsequent iron-sulfur cluster protein loss, which leads to proteotoxic stress and ultimately cell death. To better demonstrate the cuproptosis pathway, an illustration from a recently published paper is provided in Figure 4 7).
in 1972 to the first report of cuproptosis in 2022, research into regulated cell death has made great progress. Despite this, there are still many unanswered questions, and we believe there are many discoveries and achievements to be made in the future. Going forward, Dojindo Laboratories is committed to keeping up with the trends in the field and introduce new research tools that contribute to the growth of regulated cell death research.
【参考文献】
- J. Kerr, A. Wyllie and A. Currie, “Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics”, Br. J. Cancer, 1972, 26, 239-257.
- D. Tang, R. Kang, T. V. Berghe, P. Vandenabeele and G. Kroemer, “The molecular machinery of regulated cell death”, Cell Res., 2019, 29, 347-364.
- S. W. Lowe, E. M. Schmitt, S. W. Smith, B. A. Osborne and T. Jacks, “p53 is required for radiation-induced apoptosis in mouse thymocytes”, Nature, 1993, 362, 847-849.
- S. J. Dixon, K. M. Lemberg, M. R. Lamprecht, W. S. Yang, B. Morrison III, B. R. Stockwell, “Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death”, Cell, 2012, 149, 1060-1072.
- T. Shimomura, N. Hirakawa, Y. Ohuchi, M. Ishiyama, M. Shiga, and Y. Ueno, “Simple Fluorescence Assay for Cystine Uptake via the xCT in Cells Using Selenocystine and a Fluorescent Probe”, ACS Sens., 2021, 6, 2125-2128.
- P. Tsvetkov, S. Coy, B. Petrova, M. Dreishpoon, A. Verma, M. Abdusamad, J. Rossen, L. Joesch-cohen, R. Humeidi, R. D. Spangler, J. K. Eaton, E. Frenkel, M. Kocak, S. M. Corsello, S. Lutsenko, N. Kanarek, S. Santagata, and T. R. Golub, “Copper induces cell death by targeting lipoylated TCA cycle proteins”, Science, 2022, 375, 1254-1261.
- S. Li, L. Bu and L. Cai, “Cuproptosis: lipoylated TCA cycle proteins-mediated novel cell death pathway”, Signal Transduction Targeted Ther., 2022, 158.