Ferroptosis: Beauty or the Beast
 |
Zhejiang University School of Medicine & Hengyang Medical School University of South China Vice President for University of South of China(USC) |
 |
Institute of Translational Medicine Zhejiang University School of Medicine Professor in the Institute of Translational Medicine |
 |
School of Public Health Zhejiang University School of Medicine Postdoctoral Fellow |
 |
Zhejiang University School of Medicine Undergraduate |
Abstract
Ferroptosis is a newly identified iron-dependent programmed cell death, which is distinct from apoptosis, autophagy, necroptosis and pyroptosis. The major characteristics of ferroptosis is the accumulation of lipid peroxidation. Mounting evidence suggests ferroptosis plays a pathogenic role in a plethora of human diseases, such as tissue injuries, cardiovascular and neurodegenerative diseases, cancer, and inflammatory diseases. Therefore, many efforts have been made to discover ferroptosis inhibitors and activators for potential therapeutic implications. This review summarizes current progress of ferroptosis and its potential applications in the biology and medicine.
1. Basic characteristics of ferroptosis
Ferroptosis is an iron dependent and lipid peroxidation-driven form of cell death (Figure 1). Since it was discovered in 2012, the biological function and disease relevance of ferroptosis has been recognized. Although much progress has been made, the biological signaling pathways and underlying mechanisms remain to be elucidated. The intracellular iron homeostasis is tightly regulated by the hepcidin-ferroportin axis and other important proteins, such as TfR (Transferrin Receptor), metal transporter SLC39A14, Heme oxygenase-1 (HO-1) which have been associated with ferroptosis 1)-4). Under normal condition, there is a delicate balance between the oxidation and reduction of phospholipids. Ferroptosis occurs when the lipid oxidative products are accumulated. The oxidative state of cellular lipids is regulated by ACSL4 (Long-chain-fatty-acid―CoA ligase 4), LOXs (lipoxygenase), GPX4 (Glutathione Peroxidase 4), FSP1 (Ferroptosis Suppressor Protein 1) and the newly discovered mitochondrial-located DHODH 5)-7). The synthesis of GPX4 requires cystine, which is taken up by the system XC- (xCT) on the plasma membrane. By inhibiting xCT, erastin induces ferroptosis by reducing cystine uptake, which in turn decreases GSH and GPX4 levels and ultimately leads to ferroptosis 8). Iron homeostasis was modulated by RNF217, a newly identified E3 ubiquitin ligase, which mediates ferroportin degradation 9). Further studies are warranted to explore the precise mechanism of lipid peroxidation-triggered ferroptosis.
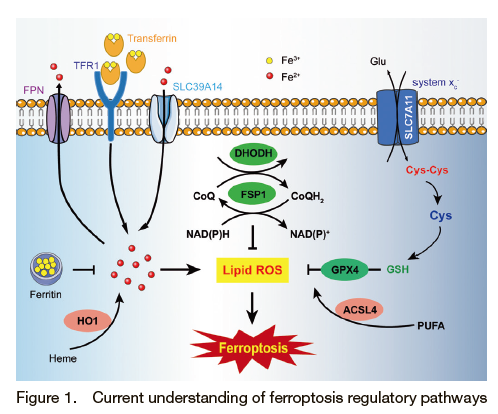
During the process of ferroptosis, cell organelles, including the mitochondria, lysosome, endoplasmic reticulum (ER) and Golgi apparatus have been reported to have functions in regulating ferroptosis. Among these organelles, the mitochondria play an essential role in regulating many types of cell death, including ferroptosis. Morphologically, the smaller size and condensed mitochondria membrane densities, the diminished/vanished mitochondria crista, and ruptured outer membrane were observed during ferroptosis 10). To date, although there is no direct evidence that the ER has a direct role in the regulation of ferroptosis, recent studies suggest that the ferroptotic modulators could induce ER stress response 11). The Golgi apparatus has been implicated in ferroptosis by regulating the cellular redox state 12). Lysosomes might be involved in ferroptosis by affecting intracellular iron through the decreased transferrin or degradation of ferritin.
Different types of cell death have different morphological characteristics. In comparison to other forms of cell death, such as apoptosis or necrosis, the ferroptotic cell death is mainly manifested as mitochondrial atrophy and damage whereas the nucleus remains intact. By contrast, typical morphological apoptosis is characterized by chromatin condensation, DNA cleavage, and nuclear fragmentation. Necroptosis-mediated cell rupture is morphologically characterized by the loss of cell plasma membrane and the swelling of organelles.
2. Targeting ferroptosis related metabolic pathways
Ferroptosis is regulated by multiple pathways and many molecules. Since it has been linked to many human diseases, enormous effects have been made to screen both agonists and antagonists of ferroptosis for the development of potential therapeutics.
2.1 Iron metabolism
Fenton reaction catalyzed by free iron in cells causes H2O2 to generate OH radicals and hydroxides, which is one of the key steps for the initiation of ferroptosis. As a result, excess free iron promotes ferroptosis whereas decreasing overloaded iron blocks ferroptosis 13). Among iron chelators, a large class of ferroptosis inhibitors, deferoxamine (DFO) potently inhibits ferroptosis agonist RSL3-induced ferroptosis, which has been widely used in a variety of diseases, including neurodegenerative diseases, cardiovascular diseases, hematologic diseases and various tumor types. Deferiprone (DFP) is the first oral iron chelator to be used in long-term clinical trials since 1980. Until 2019, our group, for the first time, identified ferroptosis as the major pathogenic mechanism for doxorubicin-induced cardiomyopathy, and Dexrazoxane (DXZ), the only FDA-approved cardiac iron chelating drug, protects against doxorubicin-induced cardiotoxicity through blocking ferroptosis of cardiomyocytes 4). On the contrary, dietary iron supplementation could enhance ferroptosis-induced heart injury.
2.2 Regulators of lipid peroxidation
GPX4 is a critical molecule to suppress lipid peroxidation and ferroptosis 14). It is a popular target to intervene ferroptosis. Dopamine is a key neurotransmitter in the hypothalamus and pituitary gland, and processes multi-functions in the nervous and immune system. It was reported that dopamine could rescue ferroptosis by stabilizing GPX4 activity, which maybe significant in ferroptosis-induced neurological diseases 15). Carvacrol, a food additive, has been widely used in food industry, which was demonstrated to inhibit ferroptosis by upregulating GPX4 expression. It is well-known that selenium is essential for GPX4 activity. Supplementation of selenium has a positive effect on restoring GPX4 activity. On the contrary, GPX4 inhibitors promote ferroptosis. Compounds, like RSL3, DPI7 and DPI10, directly act on GPX4 to inhibit its activity, thus reducing the antioxidant capacity of cells and accumulating ROS, leading to ferroptosis. FIN56 promotes GPX4 degradation and consequently induces ferroptosis.
System Xc- is an amino acid anti-transporter that mediates cystine uptake 16). It is the heterodimer protein complex composed of two subunits, SLC7A11 and SLC3A2, which coordinatively regulate ferroptosis by mediating the synthesis of GSH and GPX4 activity. β-mercaptoethanol (β-ME) inhibits erastin-induced cell death but not RSL3-induced ferroptosis, suggesting β-ME regulates ferroptosis through system Xc- but not GPX4. Similarly, N-acetylcysteine (NAC, an antioxidant) also suppresses erastin-induced ferroptosis, indicating system Xc- as its target. Erastin was the first discovered typical ferroptotic inducer to reduce the GSH levels by directly inhibiting system Xc-. In addition to the cancer treatment, erastin has also been shown to have a synergistic effect with traditional chemotherapeutic drugs. Similar to erastin, Sulfasalazine (SAS), an anti-inflammatory drug, triggers ferroptosis also through inhibiting system Xc-, although to the less extent. Sorafenib, a clinically approved multi-kinase inhibitor for the treatment of advanced carcinoma, such as hepatocellular carcinoma and renal cell carcinoma, has been demonstrated to activate ferroptosis by blocking system Xc- mediated the import of cystine.
To suppress lipid peroxidation, there are a class of compounds directly influence phospholipids rather than ferroptosis-related proteins 17). Ferrostatin-1 (Fer-1), the first identified ferroptosis specific inhibitor, blocks ferroptosis by scavenging free radicals via inserting into the hydrophobic phospholipid bilayer. Due to the poor physicochemical properties and metabolic instability of Fer-1, many efforts have been made to optimize the structure of Fer-1. Compared to Fer-1, UAMCs are a series of optimized Fer-1 like compounds with better metabolic kinetic characteristics and water solubility. Among these UAMCs, UAMC-3203 is the most potent lipophilic basic compound with improved pharmacological properties 18). Liproxstatin-1 (LIP-1) was also found as a potent ferroptosis inhibitor containing amide and sulfonamide subunits through screening small molecule compounds 19). Compared to Fer-1, LIP-1 has the same mode of action but with better physical and chemical properties. Therefore, LIP-1 showed better stability, drug absorption and distribution in vivo. In addition, tocopherol (vitamin E), suppresses ferroptosis through inserting into phospholipid bilayer by trapping free radicals.
3. Pathogenetic role of ferroptosis in human diseases
Heart and liver diseases remain one of the leading causes of death in China. There is an unmet medical need to discover novel targets to prevent and treat heart and liver diseases. It is known that the death of cardiomyocytes and hepatocytes is an important pathogenic factor in the development of heart and liver injuries. As shown in the Figure 2, our team (Dr. Fudi Wang & Junxia Min) has been working on understanding and targeting ferroptosis in metabolic diseases, such as liver and heart diseases. During years of efforts, our team has made series findings not only elucidated iron overload-induced ferroptosis as the leading mechanism for triggering various liver and heart diseases, but also demonstrated that targeting ferroptosis as a potential effective strategy for treating these diseases in animal disease models. These discoveries have been well recognized as the breakthrough and milestone in the field of novel targets for the prevention and treatment of heart and liver diseases.

3.1 Heart injuries
Dysregulated iron metabolism plays an important role for heart damage. Our previous meta-analysis of human cohort studies reported that dietary heme iron intake is significantly related to the increased risk of heart disease in a dose-dependent manner 20).
To our knowledge, we are the first to demonstrate blocking ferroptosis, such as Ferrostatin-1 (Fer-1), could significantly prevent the DOX-induced cardiotoxicity by screening cell death inhibitors in mice 4). To identify the key regulators of ferroptosis in the DOX-induced cardiomyopathy model, we performed RNA-seq and found that upregulation of Hmox1 might be involved.
The activation of Hmox1 was found to induce ferroptosis by mediating the release of free iron ions from heme and accumulated in cardiomyocytes. Further functional studies have confirmed that iron accumulation and ferroptosis mainly occurred in the mitochondria of cardiomyocytes upon treatment with DOX. Notably, ferroptosis inhibitors can also significantly reduce the heart damage caused by ischemia-reperfusion, and provided promising novel strategies for the prevention and treatment of clinical myocardial infarction and other related heart diseases.
Recently, our group functionally characterized the important function of ferritin H (Fth) in cardiac ferroptosis cardiomyocytes, which published on Circulation Research 21). Fed with a high-iron diet, Fth-deficient in cardiomyocytes caused severe cardiac injury and hypertrophic cardiomyopathy by ferroptosis.
Mechanistically, downregulation of SLC7A11 in Fth-deficient cardiomyocytes through exacerbated ferroptosis via the decreased cystine and GSH.
3.2 Liver damage
In 2017, our team published a cover paper entitled “Characterization of Ferroptosis in Murine Models of Hemochromatosis” in Hepatology 22), which was the first to reveal that the in vivo function of Slc7a11 in the process of ferroptosis, and ferroptosis as an important pathogenic mechanism in iron overload induced liver injury by using multiple hemochromatosis gene knockout mouse models.
Transferrin (Trf) serves as one of the iron-containing proteins in the blood circulation, which is mainly secreted by the liver. It binds and transports ferric iron from the blood circulation to various tissues and organs. We demonstrated that hepatic Trf had an important regulatory function in systemic iron homeostasis 3). Hepatic Trf knockout mice were more susceptible to ferroptosis-induced liver injuries. Interestingly, inhibition of either ferroptosis or Slc39a14 could potently rescue Trf-deficiency induced liver injuries in mice, providing a possible therapeutic strategy for preventing ferroptosis-induced liver fibrosis.
3.3 Other ferroptosis-related diseases
In addition to the organ injuries, ferroptosis has been indicated to play a role in many other pathological processes. Activation of ferroptosis is a promising strategy in tumor treatment 23)-25). For example, many studies showed that erastin analogs and nanoparticles potently suppressed xenograft tumors in mice by inducing ferroptosis. Importantly, ferroptosis has also been shown to increase efficacy of tumor immunotherapy by accelerating ferroptosis of tumor cells 26).
Many neurological diseases are closely related to ferroptosis 27). It has been shown that accumulated iron and lipid peroxidation are related to a variety of neurological diseases, with the decreased levels of GSH and GPX4. GPX4-knockdown leads to neurodegenerative disorders and neuronal loss in mice, which could be rescued by ferroptosis inhibitory function of vitamin E. Additionally, inhibiting ferroptosis in neurons could effectively improve the prognosis of Alzheimer's disease. As an inhibitor of ferroptosis, DFO has also been shown to have a protective effect on neurons in patients with early Parkinson's disease. Further studies are needed to explore the underlying molecular mechanism of ferroptosis in neurodegenerative diseases to develop novel therapeutic strategies.
4. Future perspectives on ferroptosis research
As a newly defined form of cell death, ferroptosis is emerging as a target for many diseases. The number of ferroptosis-related research papers drastically increased since the “ferroptosis” was firstly reported in 2012. Drs. Fudi Wang and Junxia Min groups have been awarded the TOP 10 Zhejiang University Academic Advances 2020, which entitled “Identification of ferroptosis as a target for heart and liver diseases” (Figure 3). It is exciting that we organized a new book entitled “ferroptosis and human health” by a group of Chinese scientists will soon be published. Compared to other well-characterized apoptosis, autophagy and necrosis, the field of ferroptosis is growing very fast. It remains unknown with respect to the physiological role of ferroptosis. Considering its pathogenic roles in many diseases, the regulatory networks of ferroptosis are worthy of further investigation. In addition, the complex relationship between ferroptosis and other types of cell death in the progression of diseases also needs to be explored. Nevertheless, targeting ferroptosis holds a great promise for developing effective therapeutics for many diseases.

ACKNOWLEDGMENTS
This work was supported by research grants from the National Natural Science Foundation of China (31930057 and 31970689). Due to reference limitations, we sincerely apologize to our colleagues whose relevant work cannot be cited here.
| [ Contact ] |
| Corresponding authors: |
|---|
|
|
| Third author: |
School of Public Health Zhejiang University School of Medicine Zhejiang 310058, China. Graduation: Peking Union Medical College: Ph.D. Current position: Postdoctoral Fellow Current interests: Ferroptosis and immunity |
| Forth author: |
Zhejiang University School of Medicine Graduation: Zhejiang University School of Medicine Current position: Undergraduate Current interests: Metabolism and ferroptosis |

