
Migrasomes and migracytosis, an emerging field in cell biology
 |
Li Yu The State Key Laboratory of Membrane Biology, Tsinghua University-Peking University Joint Center for Life Sciences, School of Life Sciences, Tsinghua University. |
 |
Yang Chen  Center for Precision Medicine Multi-Omics Research, Peking University Health Science Center, Peking University. |
 |
Dongju Wang  The State Key Laboratory of Membrane Biology, Tsinghua University-Peking University Joint Center for Life Sciences, School of Life Sciences, Tsinghua University. |
Abstract :
Migrasomes are newly discovered cellular organelles generated through migracytosis. They function in active intracellular communications which are essential for multiple core biological processes. In this review, we summarize the current research progress on migrasomes, including the molecular mechanisms involved in migrasome biogenesis and the physiological significance of migrasomes. We also propose the potential functions of migrasomes and the future directions of this emerging field.
Main text :
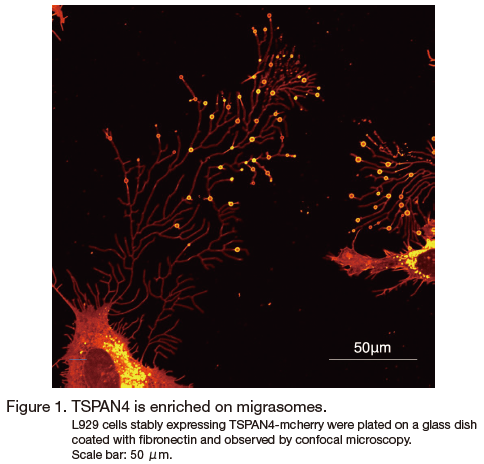
Migrasomes are newly discovered cellular organelles, first described in 2015 by Liang Ma et al 1, 2 (Figure 1). Migrasomes are vesicles with diameters on the micron scale which contain numerous small vesicles inside. They are generated on the tips or intersections of retraction fibers (RFs), the long membrane projections left by cells when they migrate away. During biogenesis, cytosolic components are actively transported to migrasomes. Once the generation process is completed, migrasomes are either broken to release their contents into the extracellular space or taken up by incoming cells. The process of migrasome release by migrating cells is called migracytosis. Thus, migrasomes and migracytosis are proposed as mechanisms for spatial and temporal cell-cell communications.
1. Molecular mechanisms of migrasome biogenesis
1. 1. Integrins provide the adhesion force for migrasome formation
Generation of migrasomes is dependent on cell migration. The number of migrasomes increases when migration is enhanced and decreases when migration is inhibited 1. In cultured cells, migrasomes adhere to the extracellular matrix (ECM) where they are formed, which indicates that adherent molecules play a role in the process of migrasome formation. Mass spectrometry analysis revealed that integrin α5β1 is enriched on migrasomes. Integrin α5β1 appears on retraction fibers before the actual growth of migrasome vesicles. Moreover, integrin α5β1 is located at the bot tom of migrasomes 3. These spatial and temporal distribution features suggest that integrin α5β1 specifies the site of migrasome formation. There are 18α and 8β integrins in mammals. Different integrins bind to different ECM proteins. Interestingly, we found that the pairing of integrins with their specific ECM partners is a determinant for migrasome formation 3. This regulatable specificity provides great potential for migrasomes to function in vivo as signal transmitters.
1.2. Tetraspanins regulate migrasome formation
Tetraspanin 4 was found to be enriched on migrasomes and has been used as a marker for migrasomes in previous studies 1, 4 . There are 33 tetraspanins in the mouse genome. Overexpression of 14 of these tetraspanins can enhance migrasome formation in Normal Rat Kidney (NRK) cells, while knockout of the highest expressed migrasome-forming tetraspanins blocks this process 5. In addition, tetraspanins and cholesterol form microdomains on migrasomal membranes. Reducing the cellular cholesterol level significantly impairs migrasome formation. Thus, tetraspanin and cholesterol are required for migrasome formation. To more accurately study the process of migrasome generation, we designed an in vitro system. Using purified tetraspanin 4-GFP (TSPAN4-GFP), cholesterol and other lipids, we generated giant unilamellar vesicles (GUVs). To mimic cell adhesion and migration, we first attached GUVs with bioengineered biotin to the bottom of a flow chamber coated with streptavidin. Then, by applying directional liquid flow in the chamber, we provided a mechanical force to move GUVs away from adherent spot. In this way, we successfully reconstituted the formation of migrasomes, and we found that both tetraspanin 4 and cholesterol colocalized on the reconstituted migrasomes. Without tetraspanin 4 or cholesterol, migrasomes could not be reconstituted; instead, small clusters of tetraspanin 4 or cholesterol were observed to be randomly distributed 5. To study the dynamics of migrasome formation in vitro, we applied a direct pulling force using a glass needle attached to the GUV instead of directional liquid flow. By pulling the GUV away with a needle, we generated retraction fiber-like structures. In this way, we observed that the initially evenly-distributed small tetraspanin 4 clusters self-organized into macrodomains, which eventually swelled to form migrasomes. Thus, the mechanical force drove the formation of tetraspanin 4 macrodomains, which in turn shaped the migrasomes. We concluded that tetraspanin 4 and cholesterol are sufficient and necessary for migrasome formation 5. Moreover, the in vitro reconstitution system provided us with an easily monitored platform to study migrasome formation with single components and made it possible to measure and calculate biophysical parameters. This made it possible to model migrasome formation. Our theoretical model explained that the shaping of migrasomes by tetraspanin 4 and cholesterol-enriched macrodomains may be explained by two biophysical properties: a high degree of bending stiffness of the migrasomal membrane and the line tension that acts at the boundary between the migrasome and the retraction fiber. The experimental parameters fit well with the calculated parameters from the modeling 5. Thus, migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. This work established tetraspanins as the key regulators of migrasome formation.
2. Physiological significance of migrasomes
2. 1. Migrasomes are essential for zebrafish organ morphogenesis
The zebrafish embryo is the first in vivo model system documented to have migrasomes 6. We found that during zebrafish gastrulation, migrasomes are formed in the extracellular pockets of space between mesendodermal cells and in the pockets between the blastodermal margin and the yolk syncytial layer. Tetraspanin 4a, tetraspanin 7, and integrin β 1b were found to regulate migrasome biogenesis in zebrafish. Knockout of tetraspanins 4a and 7 resulted in organ laterality defects including left-right reversal and bilateral duplication. Injection of exogenous migrasomes partially rescues organ morphogenesis in tspan4a and tspan7 mutants 6. Quantitative mass-spectrometry analysis showed that signaling molecules, including chemokines, morphogens, growth factors and cytokines, are enriched in migrasomes. Among these, Cxcl12 plays an important role in organ morphogenesis. We found that migrasomes were enriched around the embryonic shield and provided a source of Cxcl12a. The regional chemoattractants provided by migrasomes attract and hold dorsal forerunner cells (DFCs) at the front of the embryonic shield. The correct migration of DFCs ensures the formation of Kupffer's vesicle (KV), which is essential in establishing the left-right body axis 6. The study of migrasomes in zebrafish embryonic development provides a new mechanism for establishing signaling cues, in which signaling molecules are packed into membrane-bound compartments for release. The spatial and temporal control of signaling release adds another layer of regulation to the coordination of embryonic development 2.
2. 2. Identification of migrasomes in human serum through specific protein markers
We employed tandem mass tag (TMT) labeling followed by quantitative mass spectrometry to identify proteins enriched on migrasomes. By comparing the mass spectrometry data from migrasomes and exosomes, we identified and verified a set of specific protein markers that are enriched on migrasomes, but not on exosomes. These markers allowed us to analyze human serum samples biochemically 7. We fractionated the serum and found that a certain fraction is positive for all the migrasome markers identified. Further electron microscopy analysis confirmed the presence of migrasomes in the fraction. Thus, migrasomes are present in human serum and can be detected by western blotting using a set of protein markers 7. The identification of migrasome-specific protein markers will potentially be important in disease-related studies.
3. Future perspectives on migrasome research
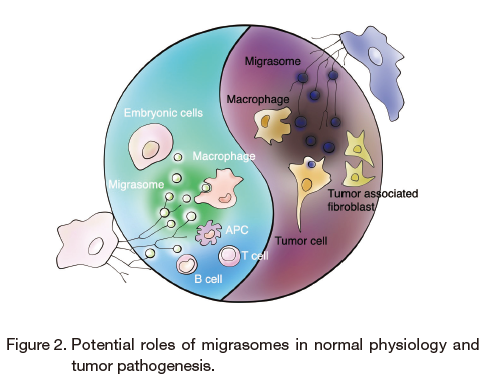
Migrasomes are emerging as a new field in cell biology. So far, we have dissected the role of integrins, TSPAN4 and cholesterol in migrasome biogenesis. Moreover, we demonstrated the essential role of migrasomes in organ morphogenesis in zebrafish embryonic development, which is the first example of the physiological significance of migrasomes. Furthermore, migrasomes were detected in human serum, which suggests their potential as vehicles for signal transduction in the circulatory system. We have already developed protein markers and a specific dye, WGA, for migrasome detection by imaging and biochemical approaches 8. However, we are still at an early stage of migrasome research. The molecular mechanisms involved in migrasome biogenesis, for example the detailed regulatory mechanisms of migrasome biogenesis, the mechanisms for sorting the enriched contents of migrasomes, and the mechanisms of migrasome uptake by recipient cells, await dissection. Moreover, we are still far from fully appreciating the biological significance of migrasomes. More extensive research to establish a deeper understanding the physiological and pathophysiological relevance of migrasomes is urgently needed (Figure 2). One direction is to establish disease models in migrasome-deficient mice to study the different phenotypes caused by defective migrasome biogenesis. On the other hand, multi-omics approaches, including proteomics, transcriptomics, lipidomics, metabolomics, and post-translationomics analysis of human biofluid samples ― for example, serum samples from large disease cohorts accompanied by data mining ― is another way to unravel the link between migrasomes and disease. Last but not least, more accurate migrasome detection methods are required for both mechanistic research and translational research in the migrasome field. Development of a highly specific, easily manipulated dye with robust signals will be very much appreciated.
Migrasomes, which are generated through migracytosis, can be considered as signal vehicles for several core biological processes, including the regulation of immune responses, embryonic cell migration, and establishment of the tumor microenvironment.
| [ Contact ] | |
| Corresponding author: | |
| name | Li Yu |
|---|---|
| Affiliation | School of Life Sciences, Tsinghua University, Beijing 100084, China. Tel : +86-(0)10-62792880 |
| Graduation | Peking University: Ph.D |
| Current position | Professor in the School of Life Sciences, Tsinghua University |
| Current interests | Migrasomes, a new organelle discovered in the Yu lab |
| First author: | |
| name | Yang Chen |
| Affiliation | Center for Precision Medicine Multi-Omics Research, Peking University Health Science Center, Beijing 100191, China. Tel : +86-(0)10-82805521 |
| Graduation | Peking University: Ph.D |
| Current position | Tenure Track Investigator in the Health Science Center, Peking University |
| Current interests | Multi-omics of extracellular vesicles |
| Second author: | |
| name | Dongju Wang  |
| Affiliation | School of Life Sciences,
Tsinghua University, Beijing 100084, China. Tel : +86-(0)10-62794552 |
| Graduation | Tsinghua University: B.S. |
| Current position | Ph.D candidate in the School of Life Sciences, Tsinghua University |
| Current interests | Migrasomes, a new organelle discovered in the Yu lab |

