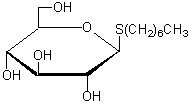
n-Heptyl-β-D-thioglucoside
Detergent
-
Product codeH015 n-Heptyl-β-D-thioglucoside
-
CAS No.85618-20-8
-
Chemical namen-Heptyl-β-D-thioglucopyranoside
-
MWC13H26O5S=294.41
| Unit size | -- | |
|---|---|---|
| 1 g | Please inquire distributors about price. | |
| 5 g | Please inquire distributors about price. | |
Description
n-Heptyl-β-D-thioglucoside is an effective non-ionic detergent for the solubilization of membrane proteins, similar to n-Octyl-β-D-glucoside. Though n-Octyl-β-D-glucoside is degraded by β-glycosidase, n-Heptyl-β-D-thioglucoside is not. This means that n-Heptyl-β-D-thioglucoside is effective for the solubilization of samples with β-glycosidase activity. The CMC value of n-Heptyl-β-D-thioglucoside is 30 mM. It is soluble in aqueous solutions at 4ºC making it suitable for the solubilization of membrane proteins at low temperatures.
Introduction
The phospholipid bilayer is the basic structure of the cell membrane. The most important functions of cells include transportation of substances, energy exchange, and transmission of information. These functions are conducted at the cell membrane by membrane proteins. In membrane biochemistry research, membrane proteins are solubilized and purified to study their structure and function. Proteins bound to cell membranes have hydrophobic sites buried within the phospholipid bilayers and hydrophilic sites facing toward the water layer. Detergents are used to isolate large insoluble molecules such as proteins. Detergents interact with the hydrophobic sites of proteins, which are then solubilized in the water layer, thus separating membrane proteins. It is important to choose a detergent that does not disrupt the bioactivities of target proteins. A detergent requires the following characteristics to be suitable for isolation of
membrane proteins:
1. Sufficient protein solubilization capability
2. No denaturing or inactivation of proteins
3. No interference with protein activities
4. No precipitation at 4ºC
5. Appropriate critical micelle concentrations (CMC) and micelle size
6. No absorption in the UV region
7. No toxicity
8. Availability of detergent detection methods
9. Non-ionic detergent if ion exchange chromatography is used
In the past, polyoxyethylene ether non-ionic detergents were widely used. These detergents, however, had several problems, such as denaturation of proteins and low CMC value, which cannot be separated easily by dialysis. n-Octyl-β-D-glucoside, n-Octyl-β-Dthioglucoside, CHAPS, and CHAPSO eliminate these problems and are widely used today. Most of the current detergents are non-ionic and easily applied to ion exchange chromatography purification. deoxy-BIGCHAP is a non-ionic detergent possessing deoxycholic acid and a gluconamide polar group. It has a high CMC value of 1.4 mM and can be easily separated by dialysis. Because its UV absorbance is low, it can be used for the determination of proteins. deoxy-BIGCHAP has been used for the extraction of opioid receptors from neuroblastoma or hybrid cells of glyoma. It has also been applied to adenylate cyclase or acetyltransferase. These detergents are also widely used to solubilize chromophores or to stabilize enzymes in diagnostic analyses and biochemical assays.
Trials of various kinds of detergents are needed to find the appropriate detergent for each study. Dojindo’s Detergent Screening Sets, which contain assorted packages of detergents, are available for use in these trials.
Chemical Structure
Technical info
Detergents are amphipathic compounds, with both lipophobic and lipophilic sites, that will form micelles above a critical concentration that is specific to each detergent. This is called the critical micelle concentration (CMC). The solubilizing abilities of detergents increase dramatically above their CMC values. After extracting membrane proteins, detergents can be easily removed by dilution, and then dialysis.
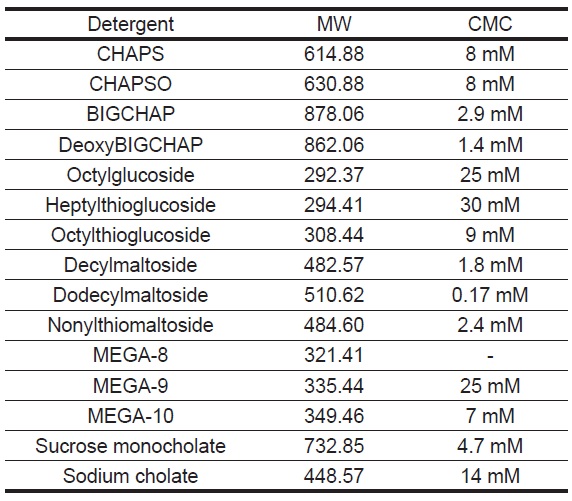
Table 1 Molecular Weight and Critical Micelle Concentration of Detergents
References
1) S. Saito and T. Tsuchiya, "Characterization of n-Octyl-β-D-Thioglucopyranoside, a Newnon-Ionic Detergent Useful for Membrane Biochemistry", Biochem. J., 1984, 222, 829.
2) T. Tsuchiya and S. Saito, "Use of n-Octyl-β-D-Thioglucoside, a New Nonionic Detergent, for Solubilization and Reconstitution of Membrane Proteins", J. Biochem., 1984, 96, 1593.
3) T. Shimamoto, S. Saito and T. Tsuchiya, "Value of Heptyl-β-D-Thioglucoside, a New Nonionic Detergent, in Studies on Membrane Proteins", J. Biochem., 1985, 97, 1807.
4) H. Itami, Y. Sakai, T. Shimamoto, H. Hama, M. Tsuda and T. Tsuchiya, "Purification and Characterization of Membrane-bound 5'-Nucleotidase of Vibrio parahaemolyticus", J. Biochem., 1989, 105, 785.
5) M. Kai, T. Yano, H. Tamegai, Y. Fukumori and T. Yamanaka, "Thiobacillus ferrooxidans Cytochrome c Oxidase: Purification, and Molecular and Enzymatic Features", J. Biochem., 1992, 112, 816.
6)T. Miki, L. S. Yoshida and K. Kakinuma, "Reconstitution of superoxide-forming NADPH oxidase activity with cytochrome b558 purified from porcine neutrophils. Requirement of a membrane-bound flavin enzyme for reconstitution of activity", J. Biol. Chem., 1992, 267, 18695.
7)Y. kashino, M. Yamashita, Y. Okamoto, H. Koike and K. Satoh, "Mechanisms of Electron Flow through the QB Site in Photosystem II. 3. Effects of the Presence of Membrane Structure on the Redox Reactions at the QB Site", Plant Cell Physiol., 1996, 37(7), 976.
8)H. Mochizuki, K. Yoshida, Y. Shibata and K. Kimata, "Tetrasulfated Disaccharide Unit in Heparan Sulfate ENZYMATIC FORMATION AND TISSUE DISTRIBUTION", J. Biol. Chem., 2008, 283, 31237.
9)H. Fujii, M. K. Johnson, M. G. Finnegan, T. Miki, L. S. Yoshida and K. Kakinuma, "Electron Spin Resonance Studies on Neutrophil Cytochrome b558", J. Biol. Chem., 1995, 270, 12685.
10)S. Hashida, S. Yuzawa, N. N. Suzuki, Y. Fujioka, T. Takikawa, H. Sumimoto, F. Inagaki and H. Fujii, "Binding of FAD to Cytochrome b558 Is Facilitated during Activation of the Phagocyte NADPH Oxidase, Leading to Superoxide Production", J. Biol. Chem., 2004, 279, 26378.
11)S. Nishida, L. S. Yoshida, T. Shimoyama, H. Nunoi, T. Kobayashi and S. Tsunawaki, "Fungal Metabolite Gliotoxin Targets Flavocytochrome b558 in the Activation of the Human Neutrophil NADPH Oxidase", Infect. Immun., 2005, 73, 235.
12)S. Okamura and S. Yamashita, "Purification and Characterizationo f Phosphatidylcholine Phospholipase D from Pig Lung", J. Biol. Chem., 1994, 269, 31207.
13)K. Ito, L. F. Li, M. Nishiwaki, Y. Okada and N. Minamiura, "Evidence for Conversion of Human Salivary α-Amylase Family A to Family B by an Enzyme Action", J. Biochem., 1992, 112, 88.
Handling and storage condition
| Appearance: | White powder or waxy solid |
|---|---|
| Purity (GC): | ≧ 98.0 % |
| Solubility in water: | To pass test (clear or almost clear, colorless) |
| Absorbance: | ≦ 0.040 (400 nm) |
| Specific rotation: | ≦ -50.0° |
| IR spectrum: | Authentic |
| 0-5°C |








